So, we set this value to the goal of our Battery 500 project. The project began in 2009 and is dominated by the Almaden Research Center. Since then, IBM has conducted this research with a number of business partners and research institutes from Europe, Asia, and the United States.
The Battery 500 project is based on metal-air technology. Compared to lithium batteries, metal-air batteries have more energy per unit mass. The project research still takes several years to be commercialized. But through these seven years of experiments, we can think that the future metal-air battery is indeed useful in electric vehicles.
Why is it a metal-air battery?
Taking lithium-air batteries as an example, to understand this problem, let's first look at the difference between lithium-ion batteries (now common lithium batteries) and lithium-air batteries.
The figure below shows the internal state of the battery during charging and discharging of the lithium ion battery. In a conventional lithium ion battery, the positive electrode is carbon, and the negative electrode is composed of different transition metal oxides such as cobalt, nickel, manganese, and the like. Both electrodes were immersed in an electrolyte in which a lithium salt was dissolved. During charge and discharge, lithium ions move from one electrode to the other. The direction of movement differs depending on whether the battery is charged or discharged depending on the state of the battery. At the time of charge and discharge, lithium ions are finally embedded in the atomic layer of the electrode material, and thus the capacity of the final battery depends on how much material can accommodate lithium ions, that is, determined by the volume and quality of the electrodes.
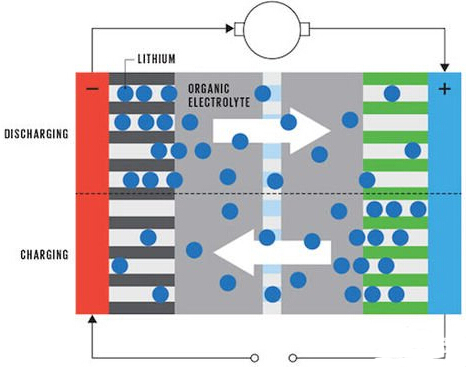
â–³ Lithium-ion battery charging and discharging process
Lithium-air batteries vary. In metal-air batteries, an electrochemical reaction takes place. During the discharge process, the lithium-containing positive electrode releases lithium ions, and the lithium ions move toward the negative electrode and react with oxygen on the surface of the negative electrode to form lithium peroxide (Li 2 O 2 ).Lithium ions, electrons and oxygen react on the surface of the negative electrode formed by porous carbon, because the chemical reaction does not occur on the negative electrode, and the lithium ion is not the negative electrode material. Therefore, the capacity of the battery and the volume or mass of the negative electrode material are not too high. Big relationship, as long as there is enough surface area.
That is to say, the capacity of the lithium-air battery is not determined by the volume and quality of the electrode, but the surface area of ​​the electrode. This is why in a lithium-air battery, a small-mass electrode can also store a large amount of energy, resulting in a higher energy density.
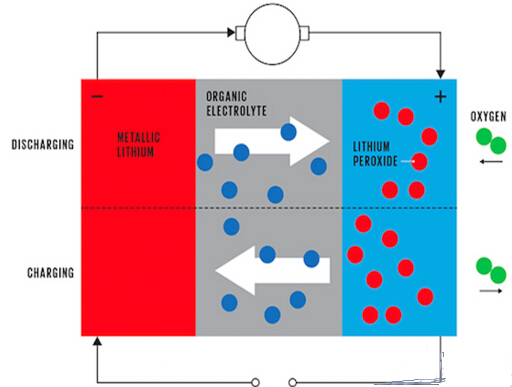
â–³ Lithium-air battery charging and discharging process
Of course, in addition to energy density, cost is also an important consideration. The price of the battery is currently in the range of 200-300 US dollars / kWh, if you can run 5-6 km per kWh, 800 km need a 150 kWh battery, you need 30,000-4.5 million. A BMW 2 Series car only needs $33,000. Therefore, if you want to mass production, the price per kWh must drop below $100.What problems should I solve for lithium-air battery commercialization?
When the lithium and oxygen are simply subjected to a redox reaction, the theoretical maximum energy density that can be produced is 3,460 Wh/kg. Aside from the portion of the cell that does not undergo a chemical reaction, the value of the energy density that can ultimately be achieved is also very desirable. Of course, you will also encounter problems.
The charging process of a lithium-air battery is similar to that of a conventional lithium ion battery, as long as it is externally pressurized. The difference is that in a lithium-air battery, when there is an external voltage, the structure of the lithium peroxide is destroyed, and it is reduced to oxygen and lithium ions, and the lithium ions are returned to the positive electrode. Lithium-air batteries, like traditional lithium batteries, have more charge and discharge cycles and have more side effects inside the battery. These side effects are fundamental to their mass production and even commercialization.
To understand the effects of these side effects on the battery, we used the electrochemical mass spectrometer at the research center to accurately measure the amount of gas consumed and produced during each charge and discharge cycle. As a result, a problem has been discovered: the lithium-air battery emits much less oxygen during charging than the oxygen consumed during discharge. (In the test, dry oxygen is used instead of air.)

â–³ IBM Research Center's electrochemical mass spectrometer (: IBM)
In an ideal battery cell, the oxygen consumed during discharge is equal to the mass of oxygen released during charging. But the study found that the amount of oxygen released is less, which means that the oxygen that is not released is likely to react with the components in the battery unit, such as melting into the electrolyte, the battery is inside. Consumption.In another IBM laboratory in Zurich, we conducted new experiments to track and computerize this self-destructive chemical reaction. Finally, the reason was found on the organic electrolyte. Then we studied this problem. In the latest battery unit, after using a new electrolyte, it can release most of the oxygen absorbed during discharge. In addition, we also track the consumption and production of hydrogen and water during charge and discharge, because the presence of these two substances means that there is likely to be at least one self-consumption chemical reaction inside the battery. Our current battery unit has been able to achieve 200 charge and discharge cycles, although this is to make the actual charging process far less than the theoretical maximum.
In addition to this problem, we have some key findings about the various components of the lithium-air battery:
1. The positive electrode is different from the positive electrode made of graphite in the traditional lithium ion battery. In the lithium-air battery, the positive electrode containing lithium will change some surface during the charging process, and some moss-like or tree-like structure grows. It is a dendrite. These dendrites are very dangerous because they can form a conductive loop between the positive and negative electrodes to create a short circuit.
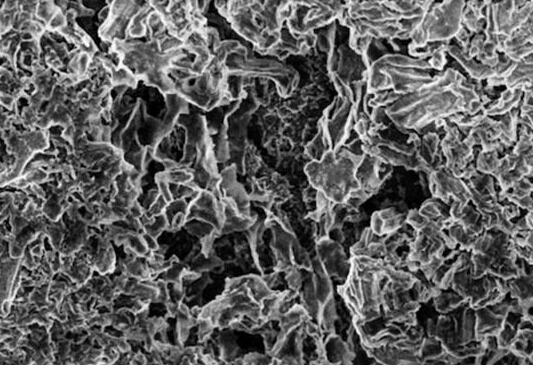
â–³ Lithium-air battery positive electrode, after several tens of cycles, the surface produces dendritic structure
In order to reduce the occurrence of dendrites, we used a special isolation membrane. This separator consists of a layer of material containing many nanoscale pores that are small enough and evenly distributed across the membrane to allow passage of lithium ions and to suppress dendritic production. Because of the presence of this separator, the anode remains smooth after several hundred charge cycles. If a traditional separator is used, dendrites will occur after several cycles. If you use a glass polymer with conductive ions, the effect will be better.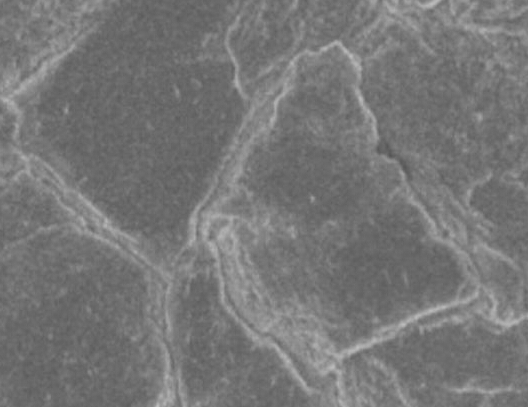
â–³ Lithium-air battery positive electrode, after using nano-isolation film, the surface remains smooth
2. The electrolyte currently used in the electrolyte still reacts with oxygen or other compounds produced in the charge and discharge cycle and is thus consumed. So far, we have not found any solvent that is stable enough to allow the lithium-air battery to enter the commercial stage.3. During the charging process, lithium ions may react with the negative electrode to produce lithium nitrate. Lithium nitrate also reacts with the electrolyte, consuming the electrolyte and producing carbon dioxide. In the test, we also tracked the amount of lithium nitrate produced and took some measures to reduce its production. However, because the required charging voltage must be higher than the operating voltage of the battery by at least 700mV. Overvoltage will reduce the charging efficiency of the battery. We have tried to convert carbon to some other metal oxides, and the results have not changed much.
4. Catalysts Regarding whether or not to use catalysts in metal-air batteries, there have been many debates between the pros and the opponents. The use of a catalyst can significantly reduce the occurrence of overpressure conditions, but the same catalyst will generally also accelerate the consumption of electrolyte. In our theoretical studies, the activation energy is very low in the oxidation and reduction of lithium. Therefore, in lithium-air batteries, the catalyst is not necessary.
5. Preparation of air Although the battery is called a lithium air battery, in fact we use dry oxygen. Emphasis is placed on "drying" because it is only necessary to remove the components of water vapor and carbon dioxide in the air. To mass-produce such air in commercial batteries, a light, efficient, and stable air purification system is needed. From this perspective, the practical application of lithium-air batteries may be in buses, trucks, and other large vehicles. Only these large vehicles can accommodate air purification equipment.
The battery unit currently used for testing is still small in size, 76 mm in diameter and 13 mm in length, which is far from enough for the standard of electric vehicles. So one of the most important tasks that needs to be done is how to make larger battery cells, package and pack many battery cells into one battery pack, and then have a battery management system. We are also testing some different sizes, such as 100 x 100mm (100mm diameter, 100mm length).
At present, this project is still in the initial basic science stage on materials and chemical reactions, but the results obtained are positive. In our study, the energy density that can now be achieved is lithium oxidoreductive reaction of 15 kWh/kg (using a raw carbon cathode, 5700 mAh x 2.7 V/g), and the energy density in the cell is approximately 800 Wh/kg.
Sodium-air battery: low energy density, but in stable metal-air batteries, there are many metals that can be used, in addition to lithium, sodium and potassium. The reverse reaction of these metals is easier, and relatively heavier metals such as magnesium, aluminum, zinc, iron, etc. have been proven to be difficult to recharge, so the Battery 500 project chose to study both lithium and sodium. metal.
Sodium-air batteries are another interesting combination, although the energy density that can be achieved is lower compared to lithium-air batteries, but its benefits are more stable.
The reason why the energy density is low is that the chemical reaction generated is different. As mentioned above, in lithium-air batteries, lithium reacts with oxygen to produce lithium peroxide (Li2O2), but in sodium-air batteries, sodium reacts with oxygen using only one electron, resulting in sodium superoxide NaO2. Instead of sodium peroxide, Na2O2. In comparison, the energy density that a sodium-air battery can produce is theoretically reduced by half, and the theoretical upper limit of energy density is 1100 wh/kg.
On the other hand, sodium-air batteries are more efficient than lithium-air batteries, and the overvoltage is quite low, less than 20mV (700mV for lithium). In view of this, the operating voltage of the battery unit can be reduced to 3V, so that the self-consumption of other components inside the battery can be reduced a lot, such as electrolyte. We measured it by experiment and got it verified. This has the advantage that the stability of the battery is quite high, and the capacity of the battery hardly changes after 50 charge and discharge cycles.
There are also some challenges in the commercial use of sodium-air batteries. For example, a sodium-air battery consumes twice as much oxygen as a lithium-air battery in response to a reaction, equivalent to the amount of air required to produce a piston engine of the same power. In addition, the chemical activity of sodium metal is quite high, and many people will remember the demonstration made by the chemistry teacher in the high school classroom. A small piece of sodium is thrown into the water, and a violent chemical reaction will occur.
However, lithium is a rare metal and it is not cheap. But sodium is a common metal and the cost is extremely low. The cost of materials in the same size sodium-air battery is less than one tenth of that in lithium-air batteries. Although in the long run, lithium-air batteries will have better performance, but considering the stability and cost, the sodium-air battery that is not as low as the energy will be a better choice from the current battery to the future.
Displacement sensor, also known as linear sensor, is a linear device belonging to metal induction. The function of the sensor is to convert various measured physical quantities into electricity. In the production process, the measurement of displacement is generally divided into measuring the physical size and mechanical displacement. According to the different forms of the measured variable, the displacement sensor can be divided into two types: analog and digital. The analog type can be divided into two types: physical property type and structural type. Commonly used displacement sensors are mostly analog structures, including potentiometer-type displacement sensors, inductive displacement sensors, self-aligning machines, capacitive displacement sensors, eddy current displacement sensors, Hall-type displacement sensors, etc. An important advantage of the digital displacement sensor is that it is convenient to send the signal directly into the computer system. This kind of sensor is developing rapidly, and its application is increasingly widespread.
Magnetic Scale Linear Encoder,Magnetic Scale Encoder,Encoder Software,Encoder Meaning
Changchun Guangxing Sensing Technology Co.LTD , https://www.gx-encoder.com