The development of electrochemical energy storage devices plays a vital role in the efficient use of new energy sources. Among them, lithium ion batteries have been widely used. However, the current energy density of lithium-ion batteries is still insufficient to meet the needs of many applications. Therefore, lithium-sulfur batteries with a theoretical energy density of up to 2600 Wh/kg have received extensive attention and research. However, in practical applications, the formation of a "shuttle effect" of polysulfides (intermediates) that are readily soluble in the electrolyte directly leads to poor battery cycle life. Therefore, how to inhibit the shuttle of polysulfide is crucial in the research of anodes of lithium-sulfur batteries.
Shuttle Effect is also called shuttle effect, which means that during the charge and discharge process, the polysulfide (Li2Sx) intermediate produced by the positive electrode dissolves into the electrolyte, passes through the separator, diffuses to the negative electrode, and directly reacts with the lithium metal of the negative electrode. Finally, it causes irreversible loss of effective substances in the battery, attenuation of battery life, and low coulombic efficiency.
In order to keep the shuttle effect, the positive electrode is physically adsorbed and impregnated with a high specific surface area of ​​a porous structure (such as graphene, carbon tube, etc.), and further chemically modified. The active site is modified to achieve chemisorption.

Shuttle effect. The shuttle effect is that the sulfur positive electrode will form a polysulfide after the electron is obtained. The polysulfide compound will pass through the separator to the electrolyte on the side of the Li electrode and react with the Li metal under the influence of the concentration gradient. LixSy goes back to the positive pole. To put it bluntly, the anode lithium is taken away, and when it is charged, it will not return to cause the loss of Li. At present, this problem is still very big. Some groups have been modified on the diaphragm, but the effect is not great, but it has caused a lot of loss to the capacity. This problem has also had a great impact on the cycle life of lithium-sulfur batteries.
Sulfur is a poor conductor and it cannot be used as an electrode. Well, the electrode is not conductive. It is impossible for everyone to find ways to make the conductive material. What carbon nanotubes, graphene, carbon black, Super P, acetylene black, and the internal combustion of sulfur, the energy density of these conductive agents is low. Ah, there are some blends that require adhesives, and the binder has a lower energy density! The advantage of sulfur as a positive electrode is not fully reflected.
This is not yet complete, the density of sulfur is smaller than the final lithium sulfide and Li2S2, resulting in a 20% change in the volume of the positive electrode, which causes problems such as the positive electrode material falling off on the current collector.
However, in fact, my own experiments are not many, only one hundred and ten, I found that this battery is not very 'stable', not to say that it will explode, the same batch of batteries, 10 batteries out of 7, 8 Different results are normal! Estimated to be a problem with my operation, although I was still very confident about my level of operation, but the result is really making me unable to determine which is the real performance of this battery.
A new strategy to effectively suppress the "shuttle effect" of lithium-sulfur batteriesLithium-sulfur batteries have become the most promising substitute for lithium-ion batteries because their theoretical energy density (2500Wh/kg) is much higher than the energy density (200Wh/kg) of existing lithium ions. However, in the Lithium-ion/delithiation process, the "redox shuttle effect" caused by polysulfides (PSs) dissolved between the sulfur positive electrode and the lithium negative electrode results in a shorter cycle life in practical applications. .
Various methods have been taken to improve the above problems, and the most common strategy is to use a nanostructured carbon material having a high specific surface area to capture PS by physical confinement. Another effective method is the capture of PS by chemical interaction using polar materials.
However, the non-polarity of carbon generally leads to poor cycle performance, and the low conductivity of polar materials results in low sulfur utilization and poor rate performance. The binding ability of carbon materials to PS can be improved by element doping, layered structure, graphene coating, etc. The conductivity of polar materials can be reduced by hydrogen (hydrogenated TIO2) or unique carbon/polar materials. Structure to improve.
However, these complicated preparation processes reduce their feasibility, so it is necessary to develop an effective material preparation method which is simple but can significantly improve the cycle performance of the sulfur positive electrode while maintaining good rate performance.
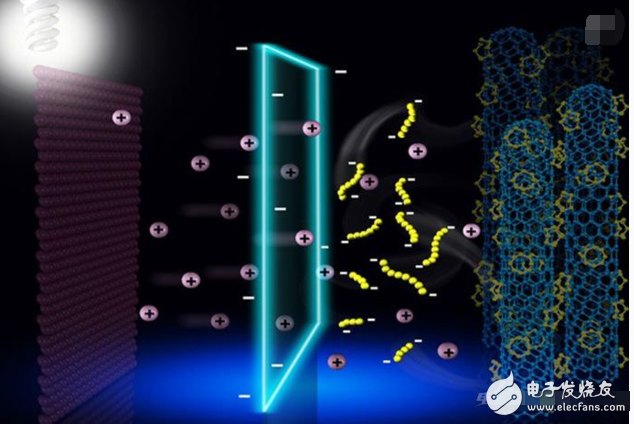
Wrapping the PS with a hermetic encapsulation layer is the most intuitive way to solve the shuttle effect of Li-S batteries. An effective packaging strategy depends on the simultaneous encapsulation of the sulfur and electrolyte barriers that allow the passage of Li+, preventing the PS from accumulating while inhibiting the migration of dissolved PSs to the negative electrode.
The negative electrode produces a solid electrolyte interface film (SEI) during charge and discharge below 1.0 V, obtaining a barrier layer on the carbon and Li negative electrode surfaces of the lithium ion battery in a very simple manner. This SEI film prevents the negative electrode from coming into contact with the external electrolyte and prevents further irreversible and unfavorable reactions between the negative electrode and the electrolyte.
At present, the physical adsorption of polysulfides with carbon materials with high specific surface area and the chemisorption of polysulfides with polar oxides are the main methods for inhibiting the shuttle of polysulfides. In contrast, confining polysulfides to a confined space is a more direct and effective strategy. However, this method has not achieved good results for a long time. This is because if only the polysulfide is fixed in a closed environment, the polysulfide cannot be brought into contact with the electrolyte to be insoluble in the electrolyte and deposited on the surface of the conductive material. Since the conductivity of sulfur and its reactants is extremely poor, the reaction of sulfur can only occur on the surface of the conductive material, so the deposited polysulfide will affect the full progress of the subsequent sulfur reaction, resulting in low utilization of sulfur. Therefore, how to seal the polysulfide and the electrolyte at the same time is a prerequisite for achieving this effect.
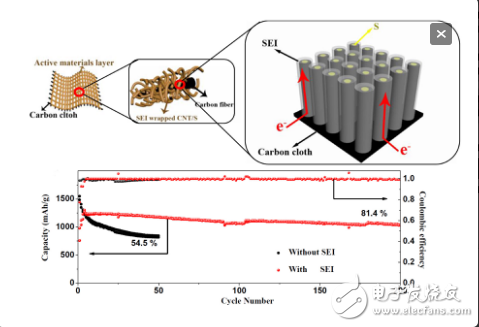
In lithium ion batteries, SEI films are often used to isolate contact of negative electrode materials (carbon, lithium, etc.) with electrolytes. Moreover, the SEI film only needs to be formed by charging and discharging several times below 1V (vs. Li+/Li), which is very convenient. Inspired by this, recently Professor Zhang Fengxiang of Dalian University of Technology and Professor Liu Jinping of Wuhan University of Technology proposed a pre-growth solid electrolyte interface membrane (SEI)-coated carbon (porous carbon sphere, three-dimensional carbon nanotube array, etc.)/sulfur composite positive electrode. , significantly improve the cycle stability of the sulfur electrode. The researchers first used SEI film as an intelligent barrier layer to seal sulfur and electrolyte into porous carbon spheres. Thus, when the positive electrode was charged and discharged, polysulfide could be dissolved but could not shuttle, effectively suppressing the shuttle effect. Moreover, the SEI film is very "smart" to both hinder the dissolution of polysulfide and allow the conduction of lithium ions, so that the electrode reaction can be sufficiently carried out, exhibiting excellent stability. Further, they extended this method to other carbon-sulfur cathodes with good morphology, and achieved good results. The three-dimensional carbon nanotubes CNT/S composite array electrode was constructed, and the coulombic efficiency and magnification of the SEI coated counter electrode were studied in depth. The effect of performance, verification and reveal the microscopic process and mechanism of SEI membrane inhibition of "shuttle effect".
Basic Physics Experiment Instrument Series
Basic physics experiment instrument series, used in physics laboratories of colleges and universities.
Basic Physics Experiment Instrument,Light And Optical Instruments,Optical Viewing Instrument,Microscope Light Source Instrument
Yuheng Optics Co., Ltd.(Changchun) , https://www.yuhengcoder.com